Sections
Nanowire battery makes use of nanowires to increase surface area of its electrodes. There are many variations of lithium-ion battery in the form of silicon, germanium and metal oxides. These variations replace the use of graphite anode and hence improve the battery performance.
Nanowire is a simple nanostructure which has a diameter of about a nanometer. This can be defined as the ratio of length to width which is greater than 1000. Its thickness can be constrained to tens of nanometers or less. Some of the different types of nanowires that exist are YBCO, Ni, Pt, Au, Si, InP, GaN, SiO2, TiO2 and molecular nanowires.
Many of the consumer electronics applications make use of lithium ion batteries. The increasing demand for better consumer electronics applications, introduced a great pressure to the battery time. Thus, many alternatives to the lithium ion technology is essential. This requirement has led to the need for nanowire batteries.
For lithum battery anodes, silicon is one of the materials that can be used. It has many material properties. Low discharge and high theoretical charge capacity are the basic properties of the silicon material which makes it good compared to the graphite anodes. By the use of nanowires, properties can be improved by increasing the surface area which is in contact with the electrolyte. This process will help in increasing the anode power density and helps in fast charging, better current delivery.
But due to the volume expansion during lithiation, the usage of silicon anodes in the batteries has been reduced. 400% of the silicon gets swell while the intercalation takes place at the time of charging process. The volume gets expanded here due to the crack propagation and moving lithiation front. The presence of cracks can lead to pulverization and capacity loss.
Nanowires based on silicon have a capacity of 4200 mAh g^-1. While coating a carbon layer to the silicon nanowires, the silicon material gets stabilized forming a stable solid electrolyte interphase (SEI). SEI is a byproduct of electrochemistry which occurs in the battery. Due to its formation, the capacity of the battery gets decreased. Also it can lead reform and dissolve the multiple battery cycles. When the carbon is being coated, it is seen that the capacity is 89% of the initial capacity after 200 cycles.
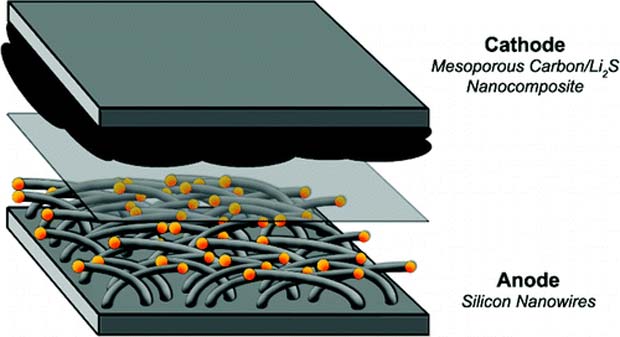
While in other design, it uses a stainless steel anode which is covered with silicon nanowires. Silicon helps in storing more lithium than graphite which leads to better energy density. Large surface area helps in increasing the anode power density, provides fast charging, and better current delivery. This anode was invented in the year of 2007.
In September 2010, the researchers showed that it maintained about 80% of the storage capacity. Studies related to silicon nanowire anodes showed that there is a fade in the energy capacity due to the volume expansion of the silicon nanowires during the lithiation process. Researchers bought out a solution to the problem in 2012 which showed that while doping the impurities to the nanowire anode helps in improving the performance of the battery. The researchers also showed that the possibility to sustain 85% of the initial capacity after the process of cycling 6000 times the undoping Si anode to a Si nanotube with Si oxide ion permeating layer as coating.
Silicon nanowire based battery provides a flexible energy source that leads in the development of a wearable technological device. This was demonstrated by the scientists at the Rice University who showed that this is possible by the deposition of porous copper nanoshells around the Si nanowire within a polymer matrix. Lithium based polymer Si nanowire battery works with a voltage of 3.4V which is flexible and scalable.
Anode which uses Ge nanowire has the ability in increasing the cycle durability and energy density of Li-ion battery. The high theoretical capacity expands during the charging process and starts disintegrating after some cycles. Ge is 400 times more effective while intercalating lithium than silicon. These anodes were capable to retain the 900mAh/g after 1100 cycles with discharge rates of 20-100?C. This performance helped in restructure of the nanowires which with the first 100 cycles to form the mechanically robust porous network.
Transition metal oxides like Cr2O3, Fe2O3, MnO2, Co3O4 and PbO2 has many advantages as the anode materials. Most of them possess high theoretical capacity, abundance, and non-toxicity as advantages.
Lead acid battery is one of the oldest and rechargeable battery cells. Usually, lead acid battery cells have small specific energy.
MnO2 is one of the best materials with its features like high energy capacity, non-toxicity and cost effectivity. While the Li ion insertion to the crystal matrix, causes volumetric expansion. These new proposed anode materials enable the battery cell to have an energy capacity of 1279 mAh g−1 with current density of 500 mA after 500 cycles. This performance is much higher than that of pure MnO2 anode or MnO2 nanowire anode cells.
Heterojunction of the many transition metal oxides helps in the performance of LIBs. This heterojunction based anode can be used in a LIB cell.
The electric car discharging time can be avoided by the battery technology being developed in carbon nanotubes.Power remote sensors, smart cards, display screens, flexible electronics, and biomedical devices.
Sections